Digital therapeutics (DTx) have been rapidly gaining momentum, and at Lux, we have been continuing to recommend engagement with DTx companies. However, those engaging with digital therapeutics companies need to be wary of falling victim to a hype-baited trap set with clinical data.
To navigate this space, there are four hurdles organizations should be aware of when it comes to the value of a digital therapeutics company: clinical validation, regulatory approval, payer reimbursement, and physician adoption.
Clinical Validation
Companies verify efficacy in treating a condition by comparing clinical results to three different metrics. They may compare their product to no treatment, to a minimum standard of care, or to the “gold standard” of treatment. For example, Cognoa, a company that develops a diagnostic and a treatment for autism, compares itself to traditional in-person therapy sessions with an autism specialist. This comparison is with the current “gold standard” in autism care.
Typically, companies face very few challenges in getting clinical validation for their product, especially validation that precedes regulatory body-required validation trials. Clinical validation is important, but it is often where the hype regarding these companies can overshadow the actual value of a company. In fact, clinical validation is the absolute bare minimum requirement for engaging with any DTx company. One way to get through some of the clinical data hype is to recognize that a good DTx company should be validated through a comparison to the gold standard of treatment in the field.

Regulatory Approval
The U.S. FDA has two pathways for a medical product to reach the market, clearance and approval. FDA clearance requires the product to be similar enough to existing products on the market as to pose no risk to consumers. In contrast, FDA approval requires that products undergo rigorous clinical safety and efficacy trials. With these pathways, many therapeutics companies try to partner with pharma companies in order to leverage their experience and understanding with regulatory bodies.
However, with the advent of the therapeutic-focused software precertification approval program, DTx is facing a new FDA approval pathway, where pharma has little experience and DTx companies must make their own way. Startups that were in more mature (for startups) stages applied to be a part of the FDA’s pilot precertification program, and those that applied were successful in gaining approval. In general, between partnerships and being part of the development of the precertification regulatory pathway, most DTx companies are successful in gaining approval.
Payer Reimbursement
More developed DTx companies are coming to discover that payer acceptance and reimbursement is key to market viability and that preparations to engage payer support need to be made in the development stage. Typically, companies partner with pharma companies to leverage traditional insurance reimbursement and distribution pathways, as in the case of Pear Therapeutics benefiting from its partnership with Sandoz. However, other companies, such as Better Therapeutics, are seeking alternative payer reimbursement pathways, looking instead to pharmacy benefit management plans or distributing directly to patients through employers as additional benefit programs.
Physician Adoption
As the oldest of the DTx have begun to mature, physician adoption is the final hurdle to consumer adoption. There are three major approaches to gaining physician adoption. Pear Therapeutics is using the pharmaceutical model, leveraging pharmaceutical distribution partnerships to make physicians aware of and willing to prescribe the product. Better Therapeutics, which develops a product for insomnia, is partnering with a pharmacy benefit plan that specifically offers a digital dispensary – effectively curating benefit-covered products in a single platform that physicians can use when making therapeutic recommendations. Kaia Health is taking a less traditional approach – the company has been targeting physicians as part of a marketing campaign, but the app is on the App Store and offers three subscription packages for patients.
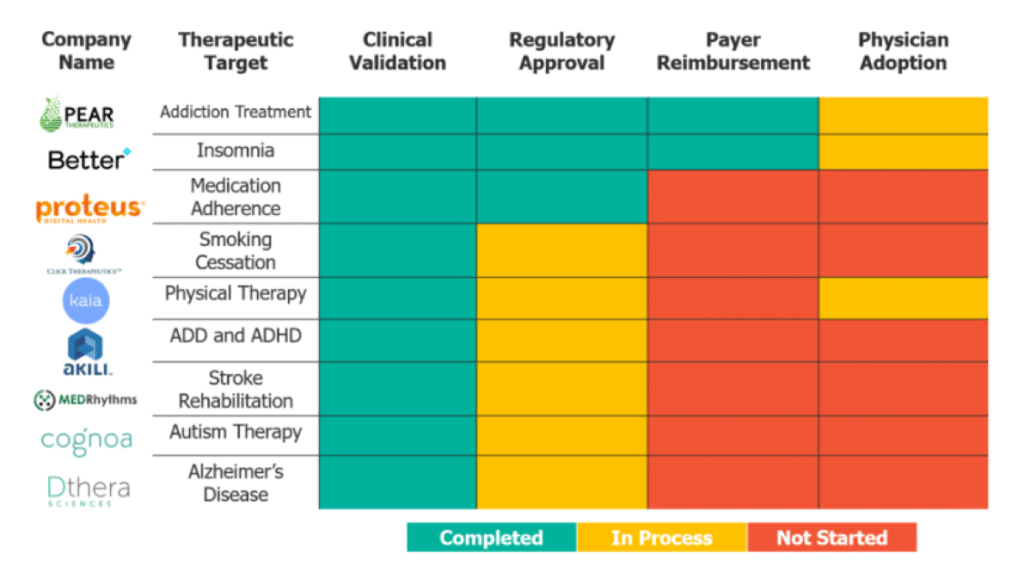
So, what does the DTx landscape look like? The figure above demonstrates where nine of the most mature DTx companies fall in terms of overcoming these four hurdles. All of the companies have acquired clinical validation of their product, and if they have not yet acquired regulatory approval, they are in the process of getting it. Of the nine, only two have navigated the payer landscape, and none of the companies have demonstrated consistent physician adoption.
With the first two hurdles (clinical validation and regulatory approval) more straightforward to overcome, there are two things to look for in a DTx company: a strategy for reaching patients, and partnerships. A reputable DTx company will have a strategy for reaching patients considering whether the therapeutic is curative, as in the case of Better Therapeutics, or designed for chronic care. Curative therapeutics tend toward a traditional insurance and physician distribution model, while chronic care tends more toward pharmacy benefits. A good DTx company will likely have at least one partnership with pharma to aid in the regulatory process, payer reimbursement, or physician adoption – few current mature companies in the DTx space lack a pharma partnership.